Add the PROPEL family of implants to your ACTION plan
The PROPEL family of implants offers 3 steroid-eluting implants under one brand, allowing ENTs to select the best option that conforms to the anatomical needs of their patients1,2
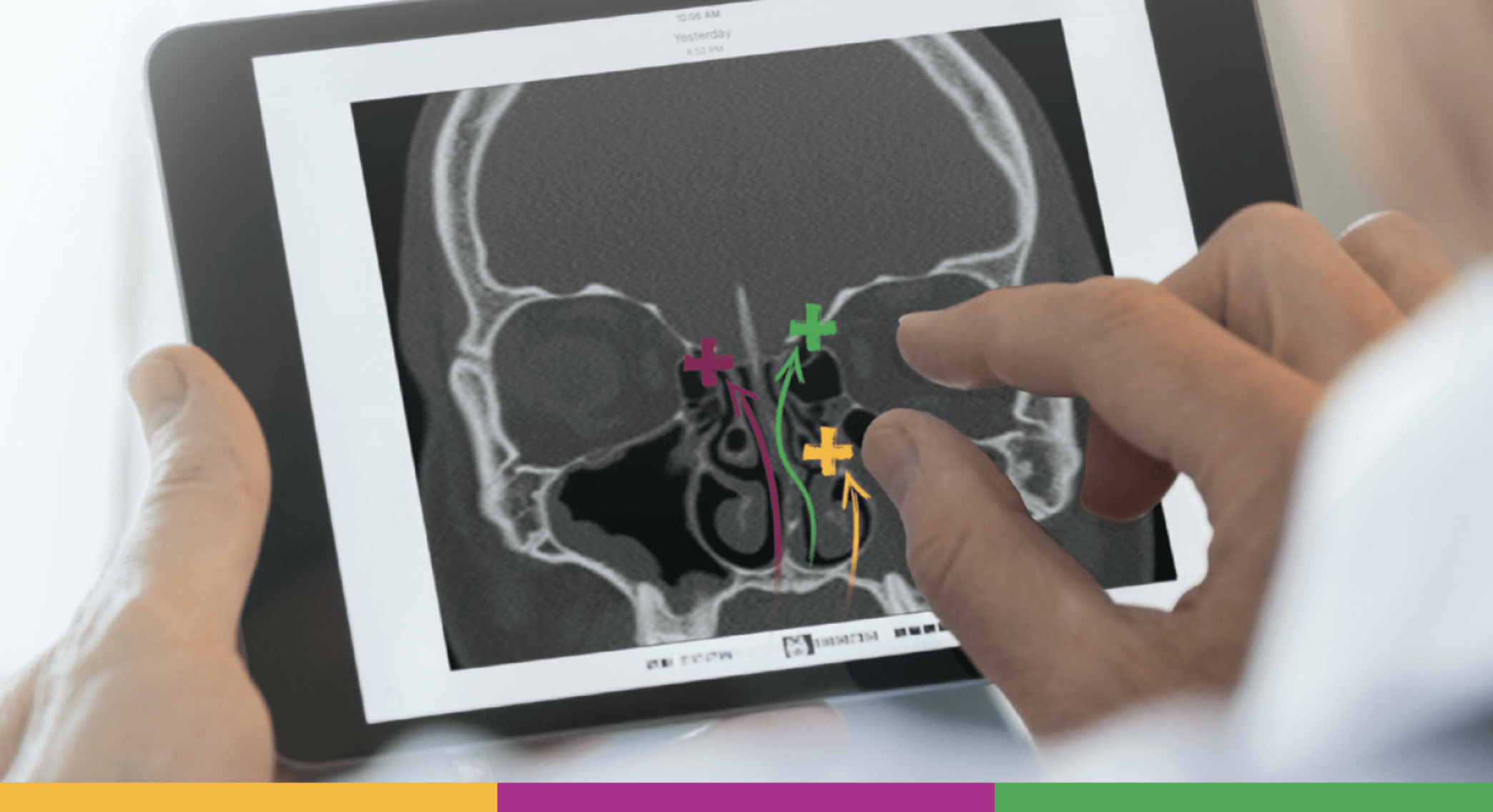
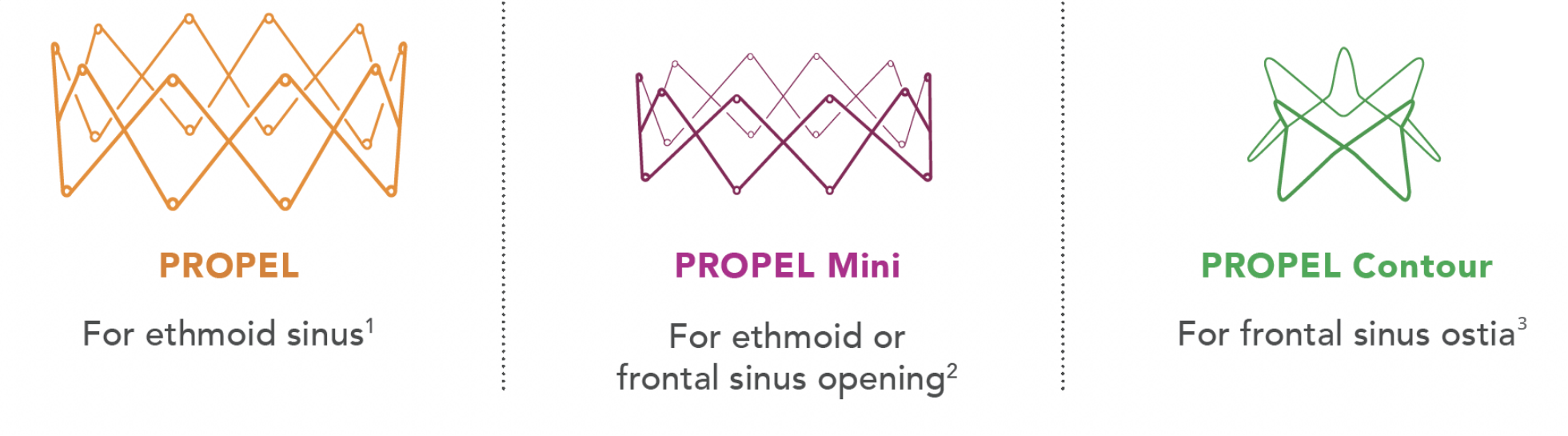
Strengthen your sinus surgery action plan with the PROPEL family of implants, an innovation in sinus technology
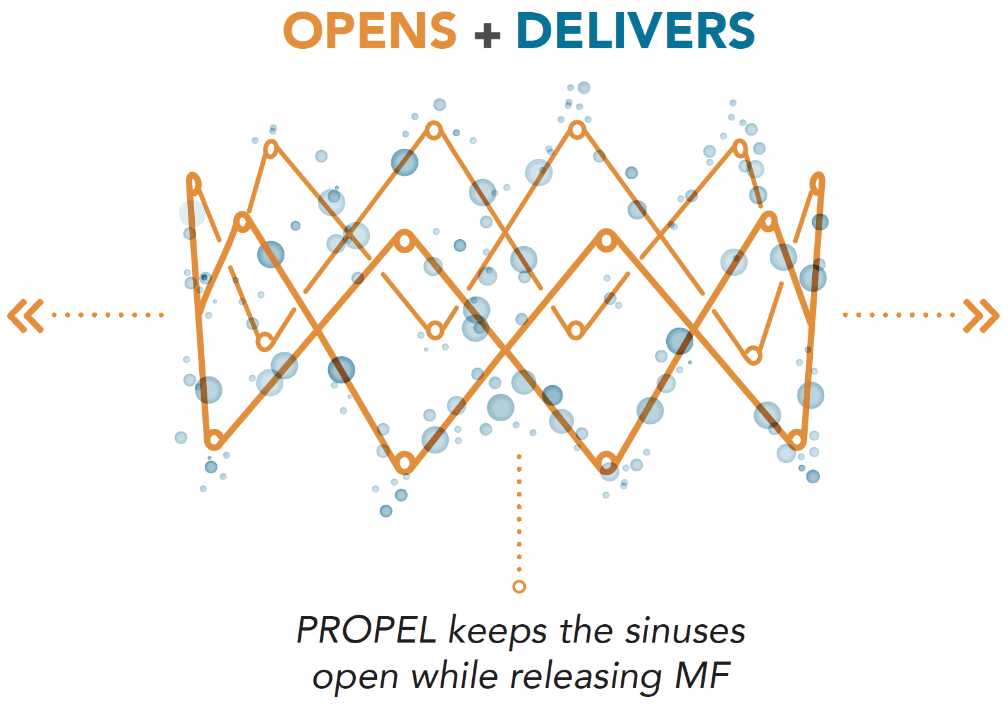
PROPEL implants feature an innovative 2-in-1 mechanism that opens in the sinuses while delivering mometasone furoate, a potent corticosteroid to the sinus mucosa, directly where it is needed1,2
OPENS in the sinus cavity
- Self-expanding design that opens in and supports the sinus cavity1
- Non-obstructive design allows for nasal clearance and the delivery of topical rinses1
- Dissolvable frame over approximately 30-45 days after placement as the sinus cavity heals14
DELIVERS Mometasone furoate (MF) locally
- Delivers 370 μg of MF over 30 days
- MF present in the mucosal tissue for up to 60 days5 (based on pre-clinical data)
MF delivers the ideal combination of potency and safety
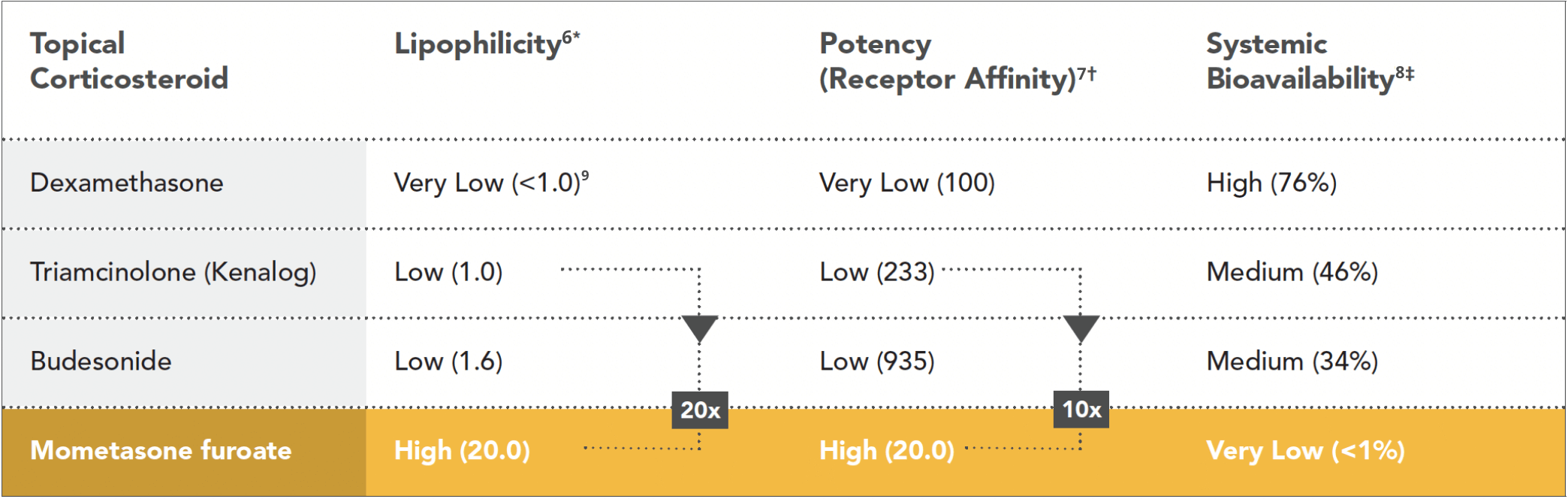
* Lipophilicity numbers normalized relative to triamcinolone acetonide.
† As measured by relative receptor binding affinity compared to dexamethasone, which is set to a value of 100. Higher values designate greater potency.
‡ As measured by plasma concentration of drug from intranasal vs intravenous route.
into tissue
receptor affinity
bioavailability
PROPEL implants are clinically proven to maintain patency and to locally deliver mometasone furoate to the sinus mucosa

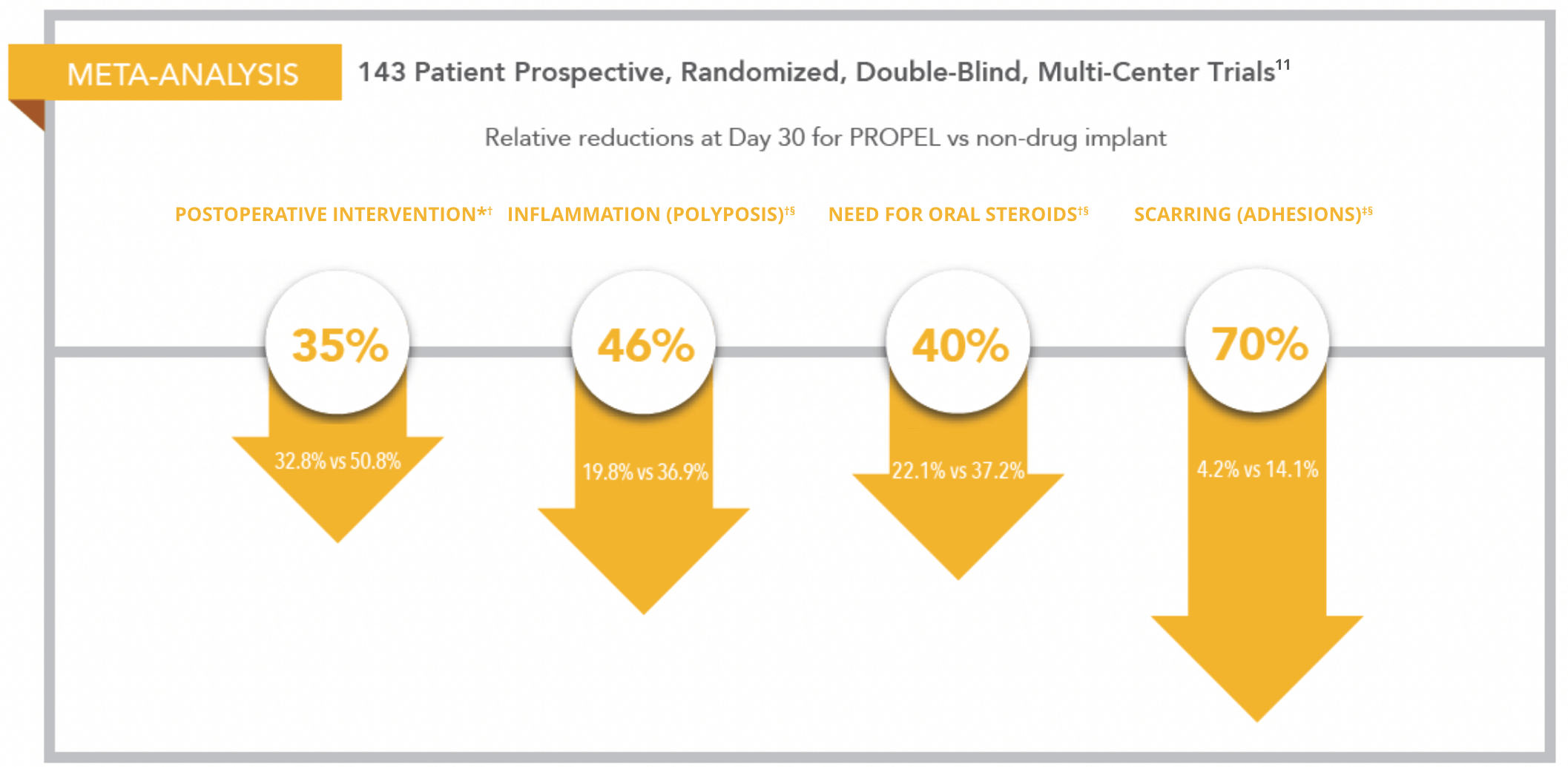
- PROPEL is the only Sinus Surgery Device Backed by Level 1a Evidence11
- 35% Relative Reduction|| in the Need for Postoperative Intervention11

- Enhances Physician Choice: Small Size, Unique Hourglass Shape, Flexible Applicator
- 65% Relative ReductionII in Postoperative Intervention12-13
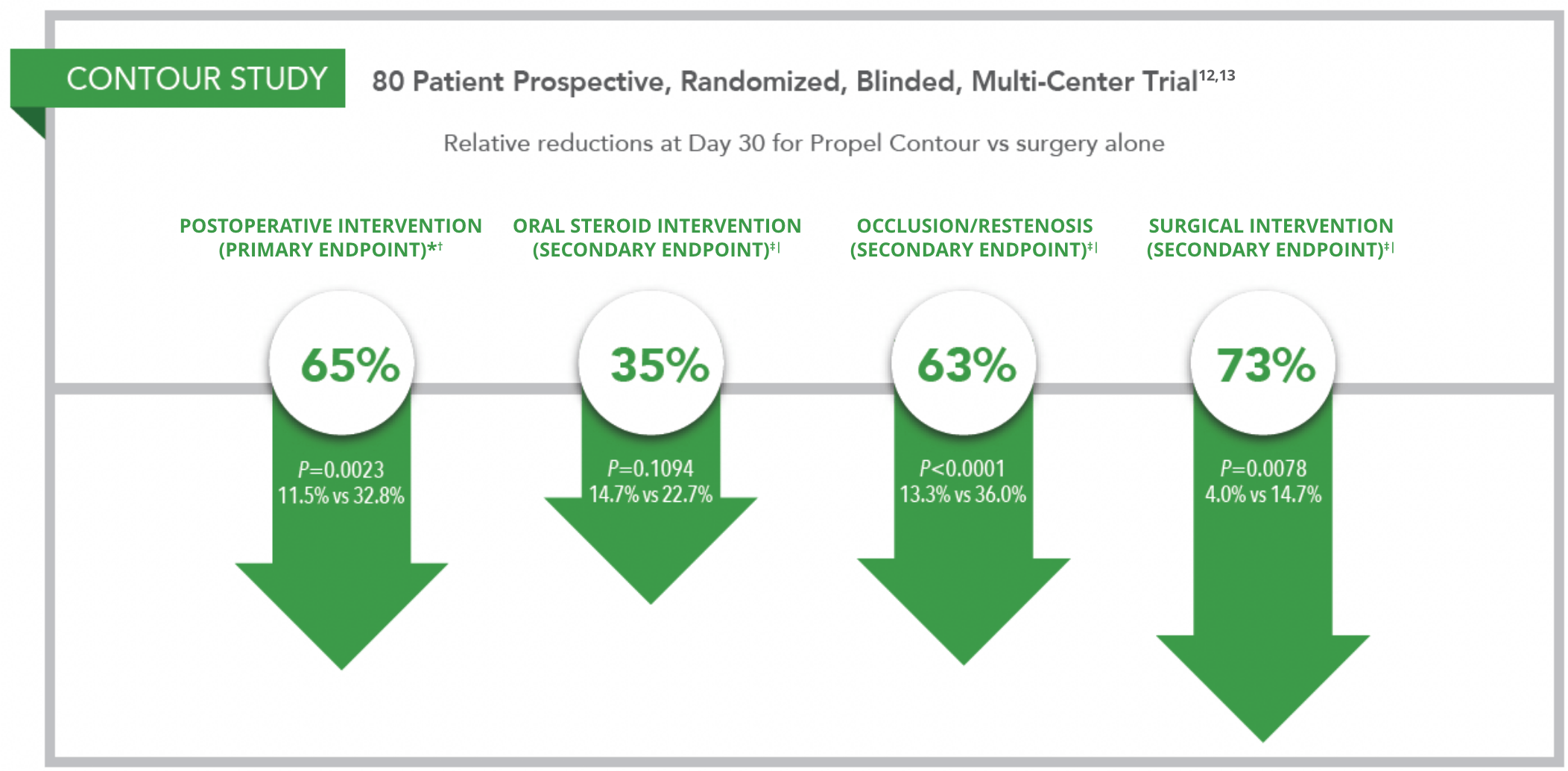
* Postoperative intervention is a composite of need for surgical interventions to separate adhesions and/or need to prescribe oral steroids for inflammation.
† Judged by an independent reviewer or independent panel.
‡ Judged by clinical investigators.
§ Secondary endpoints were not adjusted for multiplicity.
| P values adjusted for multiplicity.
|| All relative reductions Calculated as (T-C)/C as part of adhoc analysis.
PROPEL study design: Meta-analysis of two prospective, randomized, double-blinded multicenter studies (Pilot and ADVANCE II) that enrolled 143 patients.
Instructions for Use (EU):
Patient Implant Information Leaflet (EU):
References
- PROPEL [Instructions for Use]. Menlo Park, CA: Intersect ENT; 2014.
- PROPEL Mini [Instructions for Use]. Menlo Park, CA: Intersect ENT; 2016.
- PROPEL Contour [Instructions for use]. Menlo Park, CA: Intersect END; 2021.
- Food and Drug Administration. Approval Order. https://www.access-data.fda.gov/cdrh_docs/pdf10/P1000445018A.pdf. Accessed June 24, 2019.
- Li PM, Downie D, Hwang PH. Controlled steroid delivery via bioabsorbable stent: safety and performance in a rabbit model. Am J Rhinol Allergy.2009;23(6):591-596.
- Lemke T, Williams D, Roche V, Zito SW, eds. Foye’s Principles of Medicinal Chemistry. 6th ed. Balitmore, MD: Lippincott Williams & Wilkins; 2008.
- Winkler J, Hochhaus G, Derendorf H. How the lung handles drugs: pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1(4):356-363.
- Sastre J, Mosges R. Local and systemic safety of intranasal corticosteroids. J Invenstig Allergol Clin Immunol. 2012:22(1):1-12.
- PubChem. Dexamethasone. https://pubchem.ncbi.nlm.nih.gov/compound/Dexamethasone. Accessed June 24, 2019.
- Hochhaus G. Pharmacokinetic/pharmacodynamic profile of mometasone furoate nasal spray: potential effects on clinical safety and efficacy. Clin Ther. 2008:30(1):1-13.
- Han JK, Marple BF, Smith TL, et al. Effect of steroid-releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta-analysis. Int Forum Allergy Rhinol. 2012;2(4):271-279.
- Luong A, Ow RA, Singh A, et al. Safety and effectiveness of a bioabsorbable steroid-releasing implant for the paranasal sinus ostia: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2018;144(1):28-35.
- PROGRESS CSR, P500-0514, R28020, Rev 4.0, 28 December 2016.
- Murr AH, Smith TL, Hwang PH, et al. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent. Int Forum Allergy Rhinol. 2011;1(1):23–32.
Request an Intersect ENT representative
All fields required